Christie NHS Trust
Improving radiotherapy outcomes with accurate treatment simulation phantoms
Background
The Christie NHS Trust is Europe’s largest cancer treatment and research centre. Lucid has been developing medical devices with researchers at the Christie and its associated businesses for many years.
Before treatment, radiotherapy is calibrated using "phantoms", which loosely represent a body and a tumour.
Due to differences in physiology and difficulty placing sensors for cancer representation, calibration accuracy can be limited and patient throughput can be slowed. The Christie sought a more accurate and faster solution.
Objectives included:
Materials sourcing and production to accurately replicate differences in patient bone and tissue density.
Interchangeable part design enabling close simulation of patients’ anatomy.
Infinitely adjustable sensor positioning to replicate exact head and neck tumour location.
Potential to manufacture individually or in low-volume batches.
Future design adaptability to suit custom and individual patient needs.
Discovery
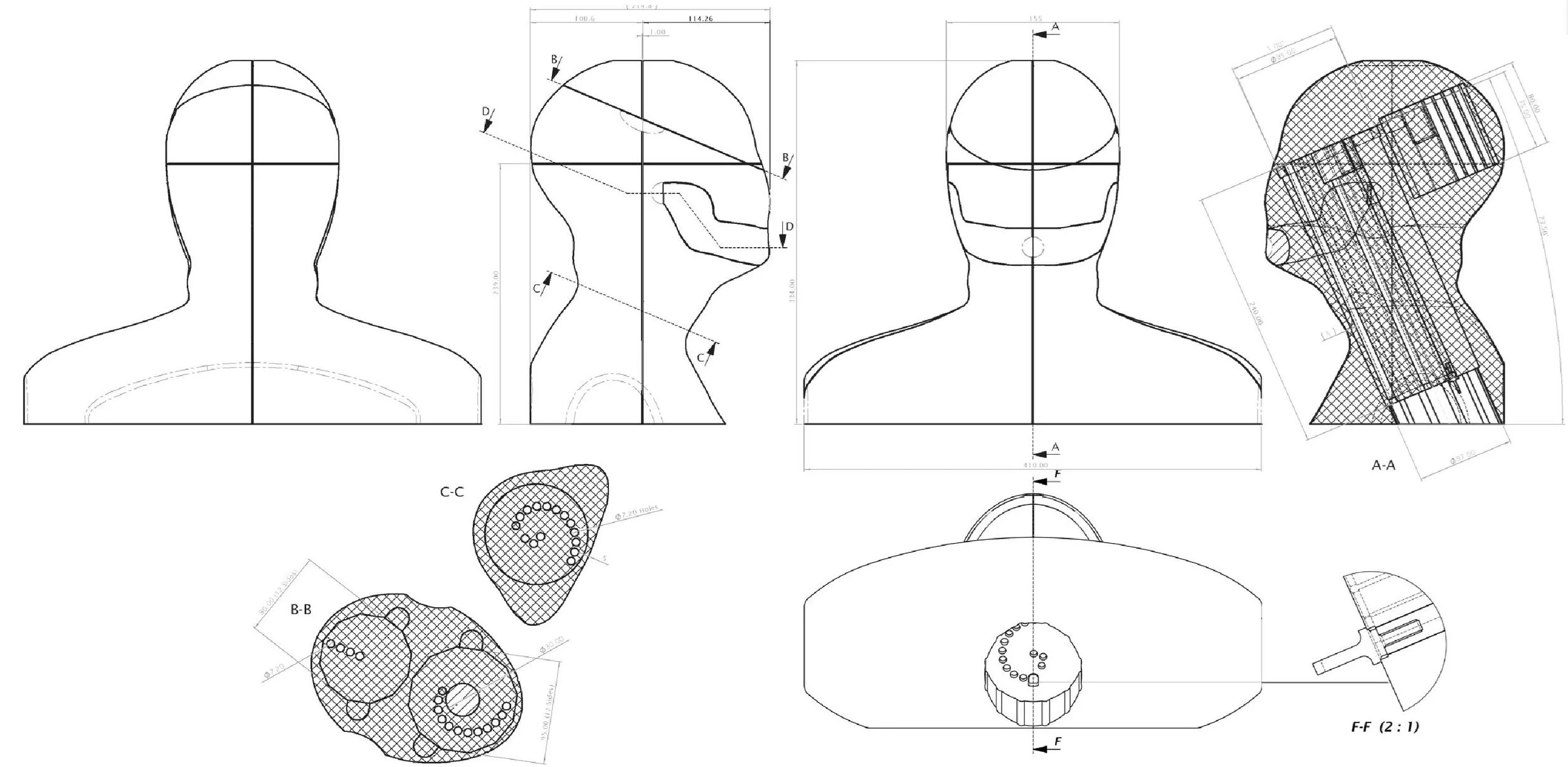
Lucid's industrial designers worked with clinicians to define sensor locations and monitor bone and tissue densities. They used body-scan data to create a 3D CAD surface model of the average head and shoulders geometry.
Design
For fast, accurate and intuitive sensor positioning in the phantom, matched to a patient’s MRI scan, a rotationally indexing insert mechanism was developed.
The phantom design can be easily customised with interchangeable parts to reflect the unique density of a patient's anatomy, as estimated by clinicians.
Delivery
Lucid delivered the whole solution from design, prototypes, specialist materials specification, and sourcing. Design and manufacture took 6 months.
Results
The Christie team has nicknamed the device “Marvin” and he continues to deliver improvement in treatment accuracy and patient outcomes.
Lucid has subsequently developed several variants to accommodate X-ray sheets and alternative sensors.
Usability
Human factors engineering includes design for simple to validate treatment setup interactions.
Regulatory
ISO 14971 risk-management and ISO 60601 medical electrical safety principles were applied throughout development to ensure integrity.
Manufacturability
The modular design has enabled straightforward and economical individual patient customisation.